Searching for niche products has always
been a problem. You have to spend much valuable time looking for a supplier,
and then you need to make sure the provider is reputable. Usually, successfully
finding a reputable, reliable, and responsible supplier of high quality
chemicals must be a headache. Relax now, because those days are over! By coming
to the LEAPChem - Pharmaceutical Chemicals
website, you have come one step closer to acquiring your trouble free
pharmaceutical chemicals. LEAPChem Highlights 2-Cyclohexen-1-one today!
Basic
Information of 2-Cyclohexen-1-one
Chemical Name: 2-Cyclohexen-1-one
Cas No.: 930-68-7
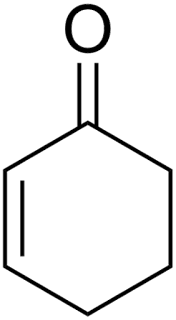
Molecular Formula: C6H8O
Chemical Structure:
Cyclohexenone is
an organic compound which is a versatile intermediate used in the synthesis of
a variety of chemical products such as pharmaceuticals and fragrances. It is
clear colorless liquid in pure state but a commercially available product is
mostly yellowish liquid.
Industrially, cyclohexenone is prepared
from phenol by Birch reduction. Cyclohexenone is a ketone, or more precisely an
enone. Common reactions include nucleophilic conjugate addition with
organocopper reagents, Michael reactions and Robinson annulations.
It is soluble in many solvents, such as
alcohols, ethers, haloalkanes, esters, and also is miscible with polar aprotic
solvents. Cyclohexenone reacts both ketones and alkenes. It has an
electron-poor carbon-carbon double bond as a typical representative of the α,
β-unsaturated carbonyl compounds. With strong bases, the positions 4 and 6 (the
two CH2-groups of the carbonyl group and the C-C double bond adjacent) are
deprotonated.
Cyclohexenone is a widely used building
block in organic synthesis chemistry, as it offers many different ways to
extend molecular frameworks. Cyclohexenone is easily adapted to Michael
addition with nucleophiles (such as enolates or silyl enol ethers) or, it could
be employed by a Diels-Alder reaction with electron-rich dienes. Furthermore,
this compound reacts with organocopper compounds from 1,4-addition (Michael
addition), or with Grignard reagents 1,2-addition, i.e., with attack of the
nucleophile at the carbonyl carbon atom. Cyclohexenone is also used in
multi-step synthesis in the construction of polycyclic natural products.
Cyclohexenone was accidentally found to be
an in-vitro catalyst for a relatively mild decarboxylation of alpha amino acids
in 1986. Researchers in Japan were attempting to use t-butyl peroxide as a catalyst
for decarboxylation using a solvent choice of cyclohexanol. Curiously they
found that when they used lower-purity (e.g. technical grade, 98%)
cyclohexanol, the reaction proceeded as much as 4 times faster compared to when
they used relatively pure cyclohexanol (>99.3%). They found that
cyclohexanol contained cyclohexenone as a natural impurity, which was three
times more abundant in the technical grade cyclohexenone compared to the more
purified cyclohexanol (~0.3% versus ~0.1%). Further research showed that 1%
cyclohexenone in cyclohexanol will decarboxylate most alpha-amino acids,
including non-standard ones, with a yield of 80-95% in a matter of several
hours. The exceptions are certain amino acids like histidine, which was
reported to take over 26 hours, and poly-amino acids, which fail to
decarboxylate using 2-cyclohexenone and another route must be found instead.
Allow a company with a ISO 9001
certification to handle all your pharmaceutical chemical needs. From finding a
reliable manufacturer who creates a quality product meeting your
specifications, to shipping to the port or facility to your choice, LEAPChem’s
experienced and professional staff will make the process as smooth as possible.
If you are interested in 2-Cyclohexen-1-one, click here to send an inquiry!
Make LEAPChem your pharmaceutical chemicals
long-term partner and contact
us today!
References:
https://en.wikipedia.org/wiki/Cyclohexenone
https://pubchem.ncbi.nlm.nih.gov/compound/2-cyclohexen-1-one
Related Articles
没有评论:
发表评论